Contact Us | 1-800-343-7475
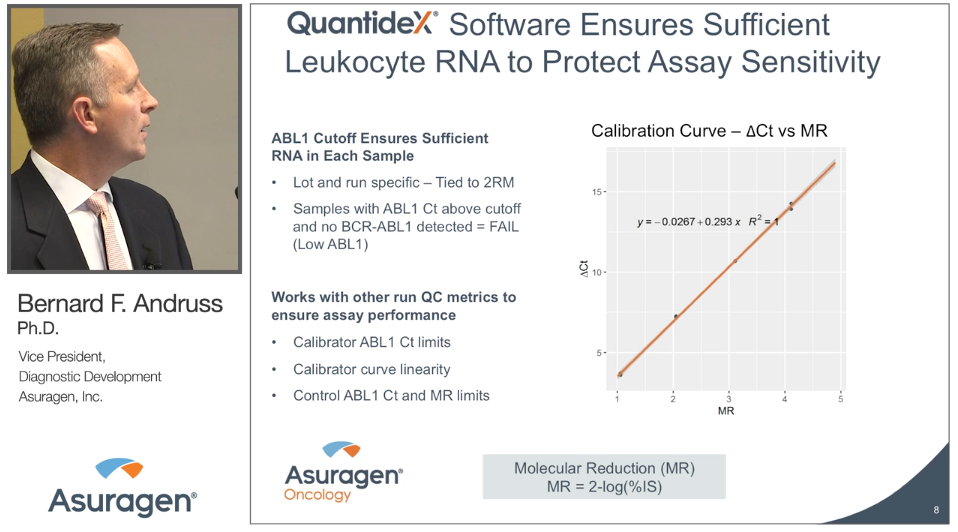
This workshop will describe the new FDA cleared QuantideX® qPCR BCR-ABL IS Kit, a highly sensitive, reproducible and clinically proven assay that detects & quantifies major breakpoint fusions (e13a2 & e14a2) on the IS scale. Our speakers will focus on both the results obtained during the FDA clinical trials and their experience in using the assay in a clinical laboratory setting with specific focus on assay performance, ease-of-use and the implications of having an MR4.7 (0.002% IS) on improving patient care.
© 2026 Asuragen, Inc. All rights reserved.